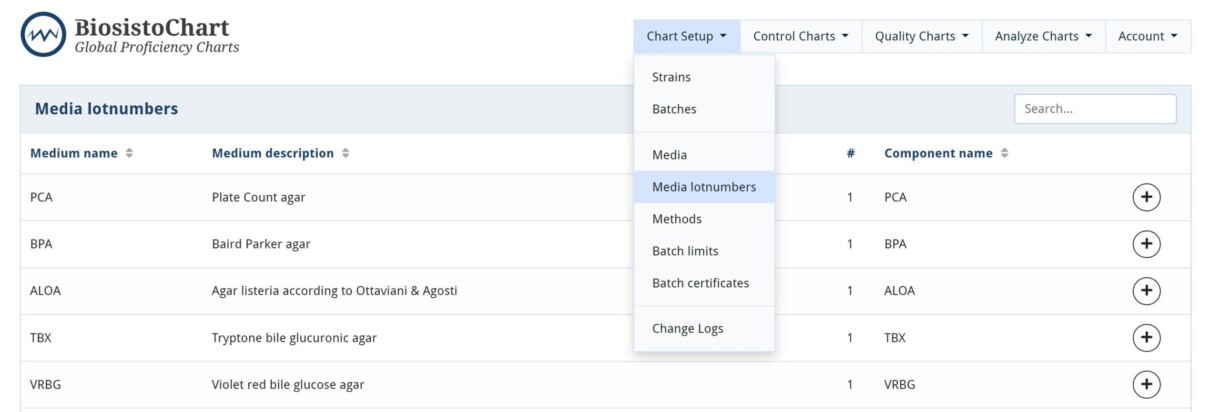
Media lotnumbers
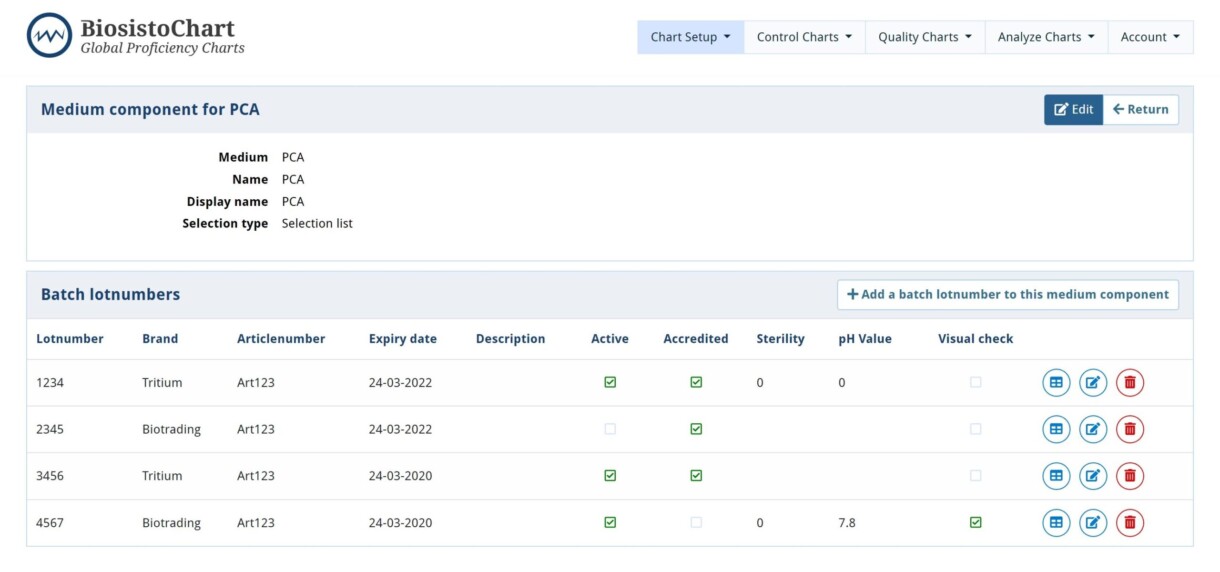
With this option, it is possible to register the used media lotnumber. This facilitates the traceability of a data point in the Control Chart to a media lotnumber. The used medium lotnumber can be selected when entering a data point in the controlChart. It also allows you to register the media checks (pH, sterility, and visual check) you performed in your laboratory.
The option for adding lotnumbers is free of fee. However, this option must be enabled. If you are interested in this option, please notify Biosisto at info@biosisto.com, and we will activate this option for you.
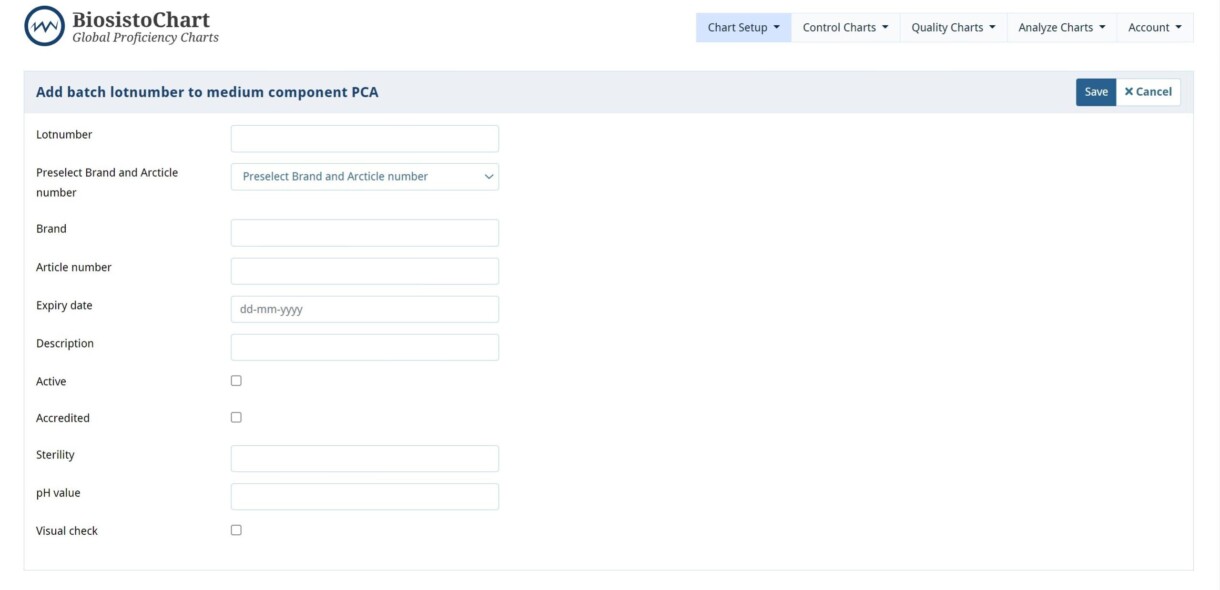
| Item | Kind of information |
|---|---|
| Lotnumber | Fill in the lotnumber of media or component |
| Preselect Brand and Article number | Choose a supplier and article number from the list if this has been used before. If not, fill in the following: |
| Brand | Fill in the name of the supplier or select from dropdown menu |
| Article | Fill in the article number of the supplier or select from dropdown menu |
| Expiry date | Fill in the Expiry date of the medium or component |
| Description | Room for extra information if needed |
| Active | Check the box if the product needs to be seen during the entry of the controlchart |
| Accredited | Check the box the medium is accredited |
| Sterility | Fill in the results of your sterility tests if performed |
| pH value | Fill in the result of your pH measurement if performed |
| Visual check | Fill in if the visual check is done if performed |







